More About Aluminum
By Cody Berry
This time, I want to talk more about aluminum. Recently, I was looking for items to use in an exhibit on WWII, and I found a few small books about the Reynolds Metals Company and the Hurricane Creek Plant which operated in Saline County for many years. In a book from 1950 called The A-B-C’s of Aluminum, I learned a few new things about aluminum production.
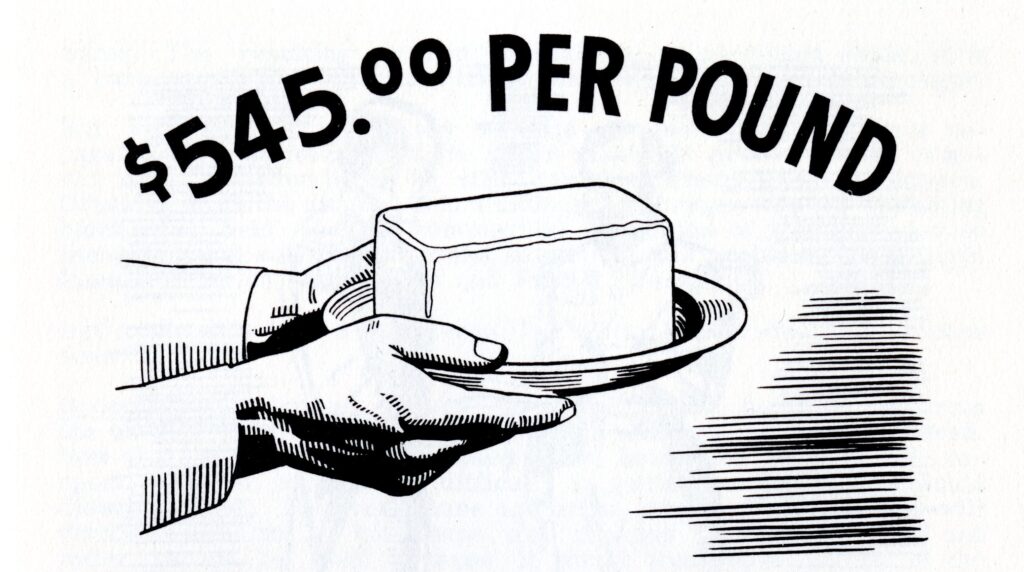
In 1825, Hans Christian Oersted, Professor of Physics at the University of Copenhagen in Denmark, produced the world’s first metallic aluminum. Two years later, a German scientist named Friedrick Woehler produced aluminum as a gray powder. In 1845, Woehler managed to produce solid aluminum particles that were about the size of a pin head. In 1852, aluminum sold for $545.00 a pound, making it, at the time, more precious than gold or silver.1
In 1854, French scientist Henri-Sainte-Claire Deville and Robert Von Bunsen of Heidelberg University worked separately discovered how to isolate aluminum by using sodium instead of potassium which led to the small particles joining to form large lumps. Deville struck off a piece of the new metal and presented it to Emperor Napoleon III who commissioned Deville to make aluminum armor and helmets for his French Cuirassiers.2
In 1886, Charles Martin Hall and Paul T. Heroult’s process, which I wrote about in my previous entry, was invented. Coincidentally both men came upon the same process at the time, but the coincidences don’t end there. Both Hall and Heroult were born in 1863 and they both died in 1914. Their process was important because it made aluminum affordable to make. As a result of the use of the Hall-Heroult Process, the price of aluminum dropped from $11.13 a pound in 1885 to just $0.57 a pound in 1892 and a record low of $0.14 a pound in 1942.3
I also learned from this book that it takes four tons of bauxite to make two tons of alumina, which only makes a single ton of aluminum. That requires a lot of energy. To make that much aluminum, about 1500 pounds of carbon electrodes are consumed as well as 20,000 kilowatt-hours of electricity. That’s enough to keep a 25-watt light bulb burning for more than 91 years!4 Aluminum was even used in paint because of its high heat reflectivity which reduces building temperatures and enhances the appearance of structures.5
Also, did you know that in the late 1940’s there was a Railroad Fair in Chicago in which Reynolds unveiled the first aluminum box car they designed? Well, they did.6 So, the reason why bauxite mining in places like Saline County was important is that it produced a metal that was cheap to produce, had many uses, and was also very strong as well. Our exhibit “How Bauxite Built A Town: 1896-1984,” pays tribute to the men and women who made it all possible.
Citations:
1 Reynolds Metals Company, The A-B-C’s of Aluminum, Louisville, Kentucky, 1950, p. 4.
2 Reynolds Metals Company, The A-B-C’s of Aluminum, Louisville, Kentucky, 1950, p. 4.
3 Reynolds Metals Company, The A-B-C’s of Aluminum, Louisville, Kentucky, 1950, p. 5.
4 Reynolds Metals Company, The A-B-C’s of Aluminum, Louisville, Kentucky, 1950, p. 8.
5 Reynolds Metals Company, The A-B-C’s of Aluminum, Louisville, Kentucky, 1950, p. 27.
6 Reynolds Metals Company, The A-B-C’s of Aluminum, Louisville, Kentucky, 1950, p. 27.




